Maintaining Sensitivity and Spectral Integrity in GC-MS: A Case Study on Hydrogen Carrier Gas Transition.
Roberto Fusetto, National Chromatography Specialist, PerkinElmer Australia
Introduction
Gas chromatography–mass spectrometry (GC-MS) remains a cornerstone analytical technique across diverse fields. Helium has been traditionally selected as the carrier gas of choice due to its inert nature and superior separation efficiency. However, helium’s rising cost and limited global availability have prompted the exploration of alternative carrier gases. Hydrogen is a compelling substitute.
With high diffusivity and low viscosity, it enables faster, more efficient separations and significantly reduced analysis times. Its cost-effectiveness and accessibility further enhance its appeal. Yet, hydrogen’s adoption is not without challenges: its flammability poses safety concerns in the laboratory, and its reactive properties complicate method transfer, often necessitating system reconfiguration, hardware modifications, and potentially compromising sensitivity and spectral matching.
This white paper presents the successful transfer of a GC-MS analytical method for quantifying terpenes in cannabis oil extracts from helium to hydrogen carrier gas. The method transfer was carried out on the PerkinElmer GC2400/MS system without any hardware modifications - no MS source change or column swap during the gas change. High purity hydrogen was supplied using the PEAK Intura H2 250 Hydrogen Generator, ensuring consistent performance throughout the study.
The evaluation focused on assessing chromatography effects, method robustness, calibration accuracy, reproducibility, and spectral integrity across both carrier gases.
Method Translation and Performance Comparison
An initial GC-MS method was developed on the GC2400/MS using helium as the carrier gas to analyze 27 terpenes in cannabis oil extracts.
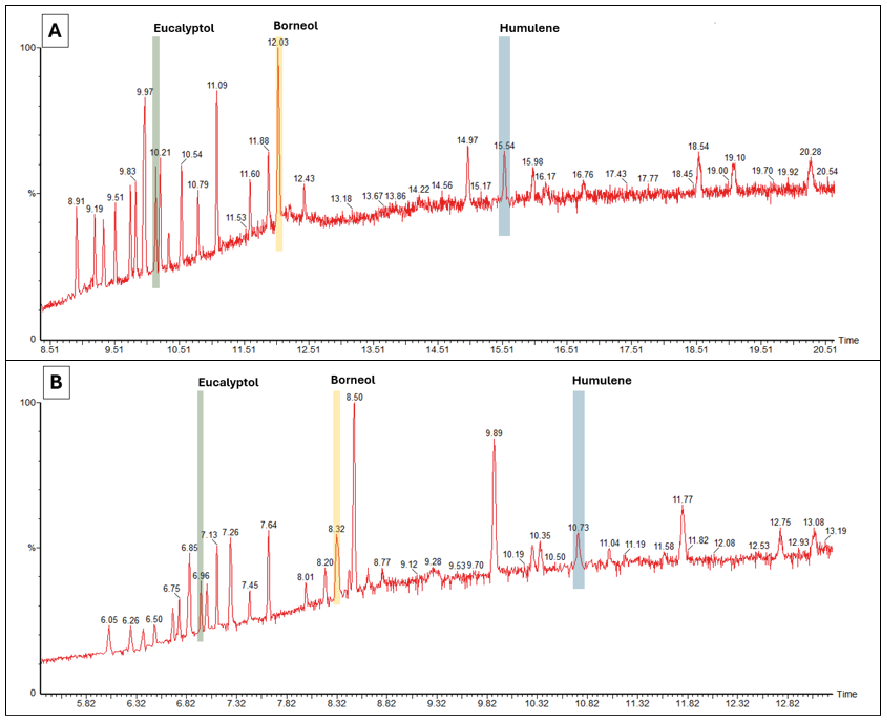
Chromatographic separation was performed on a 60-metre Elite 624 column at a flow rate of 1.0 mL/min, yielding high-resolution peaks within a 21-minute runtime. Following validation of reproducibility, sensitivity, and linearity, the method was translated to hydrogen carrier gas. The hydrogen-based method demonstrated enhanced chromatographic efficiency, achieving sharper peak resolution and reducing total runtime to 13 minutes—nearly twice as fast as the helium protocol. Three representative terpenoids—Eucalyptol, Borneol, and Humulene—were selected for comparative analysis (Figure 1). These compounds served as references for evaluating calibration linearity, injection reproducibility, and quantification accuracy under each carrier gas environment.
Calibration Curve Comparison: Helium vs. Hydrogen
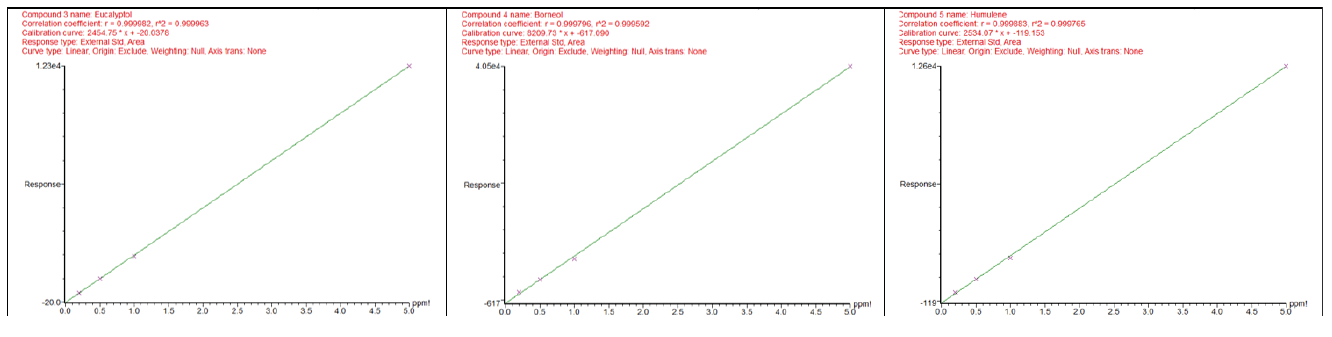
Calibration curves were constructed for three key terpenes— Eucalyptol, Borneol, and Humulene—using four concentration levels 0.2, 1.0, 2.0 and 5.0 ppm under Full MS scan mode in hydrogen carrier gas (Figure 2). The correlation coefficients (R²) for each compound were compared between helium and hydrogen carrier gases (Table 1).
| Compound | R² (He) | R² (H₂) |
|---|---|---|
| Eucalyptol | 0.999833 | 0.999963 |
| Borneol | 0.999890 | 0.999592 |
| Humulene | 0.999946 | 0.999765 |
Hydrogen significantly reduced retention times across all compounds, improving throughput. Calibration curves maintained excellent linearity (R² > 0.9995) with both carrier gases, indicating reliable quantification. Slight variation in R² values suggests minimal impact on analytical accuracy.
Reproducibility Assessment (Hydrogen Carrier Gas)
Six replicate injections of a 1.0 ppm standard were performed under Full MS mode using hydrogen as the carrier gas. Extraction Ion Chromatograms (EIC) were used to calculate the area of each compound. All compounds exhibited excellent reproducibility with RSD values below 2%, confirming method robustness under hydrogen conditions (Table 2).
| R² (He) | R² (H₂) | Humulene (m/z 93) | ||||
| Area | Conc. | Area | Conc. | Area | Conc. | |
| Rep 1 | 2326.10 | 0.87 | 6510.32 | 0.87 | 2172.34 | 0.90 |
| Rep 2 | 2330.33 | 0.88 | 6624.72 | 0.88 | 2211.25 | 0.92 |
| Rep 3 | 2272.94 | 0.86 | 6475.27 | 0.86 | 2180.47 | 0.91 |
| Rep 4 | 2297.21 | 0.86 | 6425.89 | 0.86 | 2145.1 | 0.89 |
| Rep 5 | 2252.02 | 0.87 | 6499.19 | 0.87 | 2158.81 | 0.90 |
| Rep 6 | 2246.95 | 0.84 | 6257.02 | 0.84 | 2112.82 | 0.88 |
| Average | 2287.59 | 0.86 | 6465.40 | 0.86 | 2163.47 | 0.90 |
| STD | 36.15 | 0.01 | 121.35 | 0.01 | 24.99 | 0.01 |
| RST% | 1.60% | 1.60% | 1.90% | 1.60% | 1.20% | 1.30% |
Table 2: Reproducibility testing from EIC for Eucalyptol, Borneol, and Humulene
Spectral Integrity and Library Matching
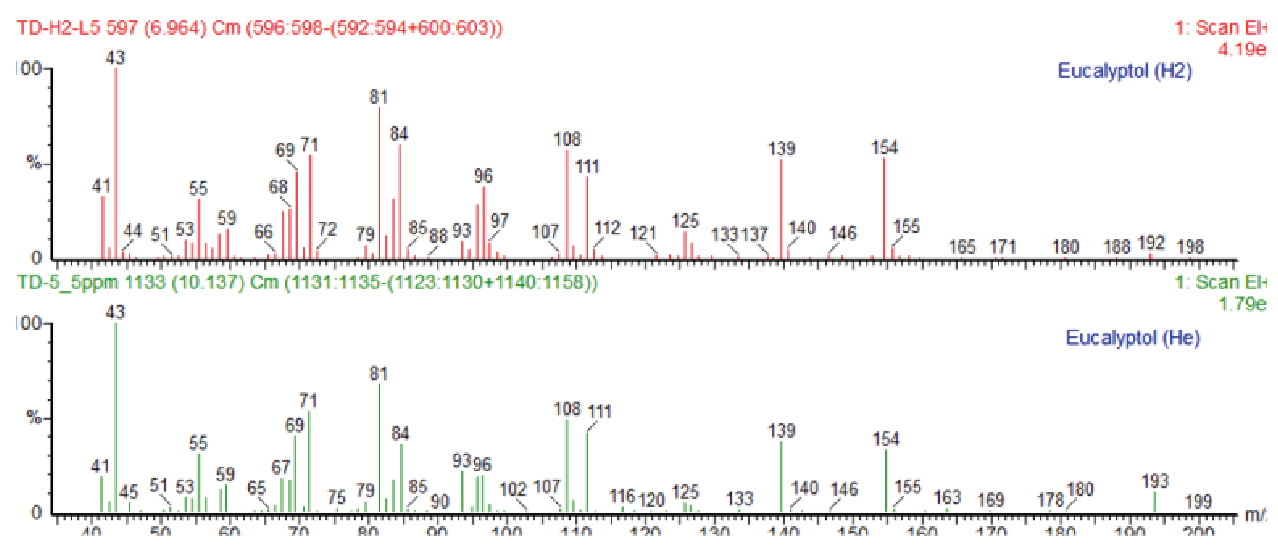
Spectral profiles obtained from a Standard solution at 5 ppm using hydrogen carrier gas were compared against those acquired with helium. The two MS spectra were compared showing close alignment across both conditions.
Furthermore, high NIST library matching values were observed for both spectra demonstrating no significant loss in spectral fidelity or compound identification accuracy. Despite hydrogen’s higher reactivity, spectral quality remained consistent, supporting its use in routine terpene analysis with minimal compromise in identification confidence (Figure 3).
Conclusion
The transition from helium to hydrogen as a carrier gas in terpene analysis significantly enhanced chromatographic performance, reducing runtime from 21 to 13 minutes while maintaining excellent resolution and reproducibility. Using Eucalyptol, Borneol, and Humulene as reference compounds, the hydrogen-based method demonstrated superior linearity and accuracy, making it a robust and efficient alternative for high-throughput terpene profiling. This optimized approach supports faster analysis without compromising data quality, offering clear advantages for routine laboratory workflows. The GC2400/MS system offers the unique capability to switch carrier gases without requiring hardware modifications—such as replacing the ion source—enabling rapid and efficient method transition and validation.
Additionally, the Intura H2 250 provides a safe, stable, and continuous supply of ultra-high purity hydrogen (99.99999%), making it the ideal choice for laboratories seeking to optimize performance while ensuring safety and reliability.
Together, these technologies streamline method development and transition, supporting high throughput workflows and robust analytical outcomes.


