Nitrogen Safety in the Lab
This blog explains why nitrogen gas generators are the safest way to supply nitrogen to your instrumentation and why they pose no danger, in terms of changing atmospheric levels of nitrogen or oxygen.
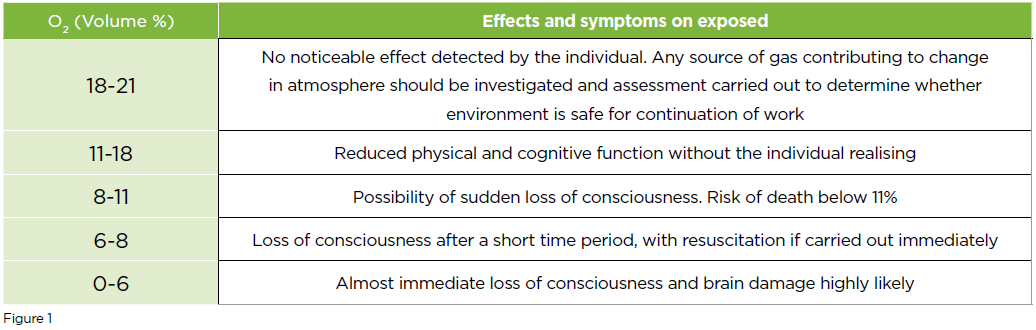
The biggest danger to lab personnel is a rapid depletion of oxygen levels in the lab. Once the level of oxygen reduces to <18% there is a risk of impaired judgement and physical ability (Figure 1). For serious risk to the wellbeing of lab personnel, the O2 content of the lab would need to be reduced below 11%. To reduce the level of O2 in the atmosphere of a laboratory measuring 3m x 3m x 2.5m, which has a total volume of 22,500L, would require 675L of N2 to be released instantaneously to decrease O2 levels by 3%, and 2250L of N2 to reduce O2 levels below 11%.
A nitrogen gas generator producing 32LPM of nitrogen would take over 20 minutes to produce 675L and over 1hr to produce 2250L of nitrogen. The 32LPM produced would at worst increase nitrogen levels by 0.14% per minute, assuming there was no air exchange within the room and this temporary separation of air has no effect on the overall air composition.
Nitrogen used by the instrument is vented back into the room, so there is no appreciable change in the overall levels of oxygen or nitrogen, the gases are only temporarily separated. Again, the amounts of gas that are temporarily separated by the generator are so small compared to the volume of air within the laboratory that the effect is negligible. In terms of nitrogen generator performance, if the oxygen content of the air in the room were to rise over time, the purity of N2 gas from the generator would gradually decline. The output from all of Peak’s nitrogen gas generators is consistent over time, demonstrating that the overall concentrations of N2 and O2 in the lab atmosphere are not changed by the gas generator.
If we contrast the amount of nitrogen that is contained in a cylinder of nitrogen (9000L) or the amount of gas that is produced from 1L of liquid nitrogen (700L), it quickly becomes apparent that a significant leak from a cylinder or spillage of liquid nitrogen poses a much larger risk to laboratory personnel, since this can cause an instantaneous change in levels of O2 in the lab atmosphere to below 11%, through loss of only 25% of a cylinder’s contents or a spillage of just 3L liquid N2.
For this reason, the NERC (Natural Environment Research Council UK) in their “Guidance on Design of Safe Laboratories” recommend a nitrogen or hydrogen gas generator. Click here for more information.
This document outlining the dangers of nitrogen from bulk supply, further illustrates the benefits of having an N2 generator in the lab from a health and safety aspect.
If you would like to know more about nitrogen safety or have any questions please contact us. You can also download and keep our Nitrogen Safety document which contains the information in this blog.
Found this article interesting? You might also be interested in: