Analysis of aromatic amines derived from Azo colorants using hydrogen as GC/MS carrier gas.
Introduction
Azo dyes are extensively used in consumer products - including textiles, leather goods, clothing, and toys - with global demand measured in millions of tonnes annually. Because many azo dyes can release aromatic amines that are allergenic or carcinogenic, the European Union has restricted or banned those azo dyes capable of yielding any of the 14 aromatic amines classified as Category 1 or 2 carcinogens under Directive 76/769/EEC. Continued assessment (initiated in 1999) is also ongoing for the remaining eight aromatic amines listed as MAK III to evaluate their mutagenic potential1. Azo dyes that can liberate any of these amines at detectable levels (above 30 ppm in finished articles or dyed parts) should not be used in textile or leather products intended for direct, prolonged contact with skin or the oral cavity2.
Using hydrogen as the GC/MS carrier gas offers practical and analytical benefits - faster analyses and improved chromatographic resolution, lower operating costs, and greater supply reliability3. This application note demonstrates the suitability of the Peak Scientific Hydrogen Trace generator as an effective carrier‑gas solution for evaluates the performance of the GCMS-QP2050 single quadrupole gas chromatography mass spectrometer for analysis of aromatic amines derived from azo colorants.
Experimental
Method Conditions
The GC/MS system was fitted with a 20 m GL Sciences InertCap 5MS/Sil column (0.18 mm i.d., 0.18 μm film thickness). Four‑level calibration standards were prepared and analyzed on a Shimadzu GCMS‑QP2020 using simultaneous scan/SIM acquisition for 26 aromatic amines; D8‑naphthalene served as the internal standard. Full‑scan spectra provided compound confirmation while quantitative data were obtained from the SIM traces.
Chromatography was run in constant linear velocity mode using hydrogen supplied by a Peak Scientific Hydrogen Trace generator. This setup achieved good peak shape and resolution and completed the separations within an 11‑minute run time.
Table 1. Analytical conditions of 26 aromatic amines compounds on Shimadzu GCMS-QP2020
|
GC conditions |
|
|
Injection Temp. |
220°C |
|
Injection Mode |
Spitless |
|
Column Flow |
0.69 mL/min |
|
Flow Control Mode |
Linear Velocity |
|
Column |
GCMS Column InertCap 5MS/Sil (20m x 0.18mm, 0.18μm), GL Science |
|
Oven Temp. |
60°C (hold 1 min) -> 25°C/min to 200°C -> 35°C/min to 310°C -> 310°C (hold 1 min) Total run: 10.74 min |
|
Carrier Gas |
Hydrogen |
|
MS condition |
|
|
Solvent Cut time |
2.1 min |
|
Ion Source Temp. |
250°C |
|
Interface Temp. |
280°C |
|
Detector Voltage |
+0.2 kV (relative to Tuning) |
|
Analysis Type |
Scan/SIM simultaneous |
Table 2. Shimadzu GCMS-QP2020 Scan/SIM acquisition parameters
|
ID |
Name |
Target SIM (m/z) |
Retention time (min) |
Ref. Ions. |
|
1 |
Aniline |
93 |
2.464 |
77.00-51.10 |
|
2 |
o-Toluidine |
106 |
3.168 |
107.00-77.00 |
|
3 |
2,4-Xylidine |
121 |
3.799 |
120.00-106.00 |
|
4 |
2,6-Xylidine |
121 |
3.799 |
106.00-120.00 |
|
5 |
o-Anisidine |
108 |
3.852 |
123.00-80.00 |
|
6 |
D8-Naphtalene* |
136 |
3.926 |
68 |
|
7 |
4-Chloroaniline |
127 |
4.032 |
92.05-65.00-129.00 |
|
8 |
1,4-Phenylenediamine |
108.1 |
4.385 |
80.10-107.10 |
|
9 |
p-Cresidine |
122 |
4.448 |
137.00-94.00 |
|
10 |
2,4,5-Trimethylaniline |
120 |
4.555 |
135.00-134.00 |
|
11 |
4-Chlor-o-Toluidine |
106 |
4.604 |
141.00-140.05 |
|
12 |
2,4-Toluendiamine |
122 |
5.088 |
121.00-94.00 |
|
13 |
2,4-Diaminoanisole |
123 |
5.558 |
138.00-95.00 |
|
14 |
2-Naphthylamine |
143 |
5.992 |
115.00-116.00 |
|
15 |
2-Amino-4-Nitrotoluene |
77 |
6.198 |
152.00-106.00 |
|
16 |
4-Aminobiphenyl |
169 |
6.911 |
170.00-115.00 |
|
17 |
p-Aminoazobenzene |
92 |
8.099 |
197.00-120.00 |
|
18 |
4,4-Oxidianiline |
200 |
8.193 |
108.00-171.00 |
|
19 |
Benzidine |
184 |
8.231 |
92.00-156.00 |
|
20 |
4,4-Diaminophenylmethane |
198 |
8.246 |
197.00-106.00 |
|
21 |
o-Aminoazotoluene |
106 |
8.626 |
225.00-79.00 |
|
22 |
3,3-Dimethyl-(4,4-Diaminodiphenylmethane) |
226 |
8.711 |
211.00-225.00 |
|
23 |
3,3-Dimethylbenzidine |
212 |
8.786 |
213.00-211.00 |
|
24 |
4,4-Thiodianiline |
216 |
8.958 |
184.00-215.00 |
|
25 |
3,3-Dimethoxy-Benzidine |
244 |
9.183 |
201.00-229.00 |
|
26 |
3,3-Dichlorbenzidine |
252 |
9.184 |
254.00-253.00 |
|
27 |
4,4-Methylen-bis(2-chloraniline) |
231 |
9.189 |
266.00-268.00 |
Note * : Internal Standard (ISTD)
Results and Discussion
In this study we used a simultaneous Scan/SIM acquisition to retain full‑scan spectral information for compound confirmation while maintaining the sensitivity of SIM for quantitation. Hydrogen was employed as the carrier gas to accelerate the run without compromising separation or resolution. The extracted ion chromatogram is shown in Figure 1, and representative mass spectra for 2,4‑toluendiamine, 2,4‑diaminoanisole, 4‑chloroaniline, and benzidine are presented in Figure 2.
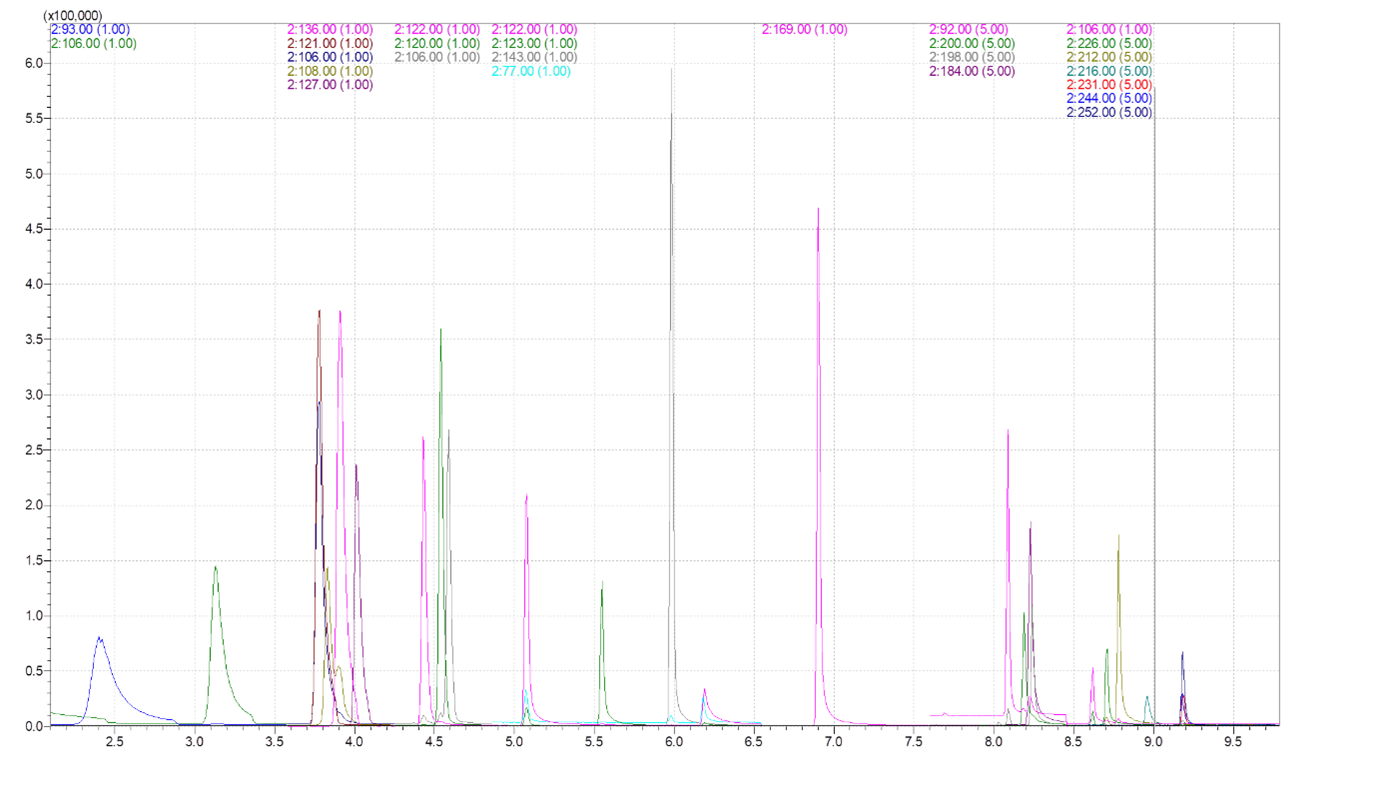
Figure 1. SIM chromatograph of 26 aromatic amines and D8‑naphthalene served as the internal standard
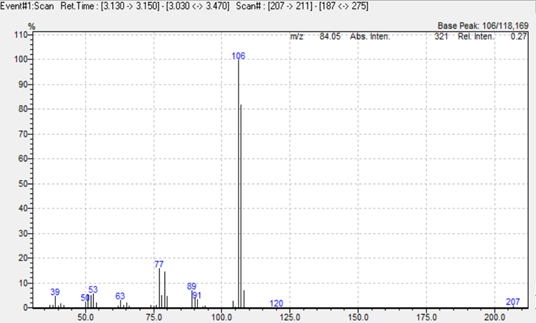
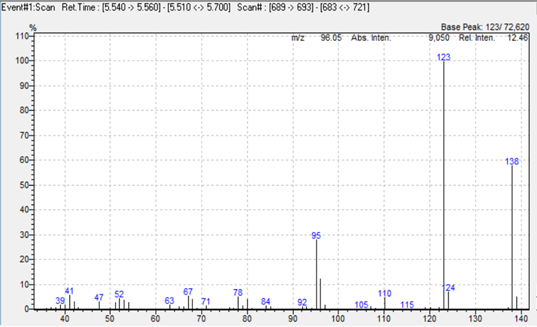
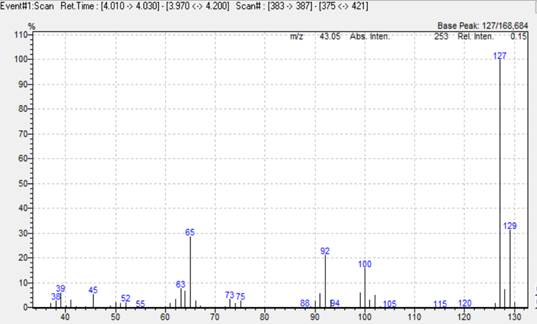
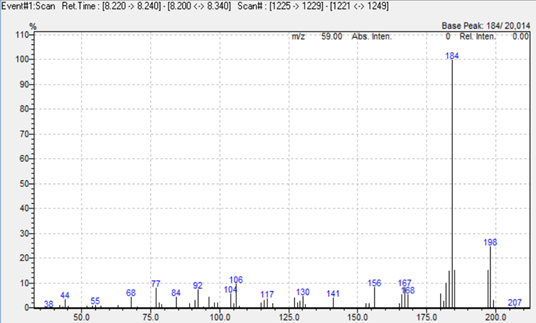
Figure 2. Mass spectra of 2,4‑toluendiamine, 2,4‑diaminoanisole, 4‑chloroaniline, and benzidine
Conclusions
This study successfully analyzed 26 aromatic amines derived from azo colorants using hydrogen as the GC/MS carrier gas. Hydrogen provided a cost‑effective alternative to helium, enabling faster analyses while maintaining chromatographic separation and resolution.
References
1. European Commission - Health and Consumers, Scientific Committees Opinion, https://ec.europa.eu/health/scientific_committees/environmental_risks/opinions/sctee/sct_out27_en.htm, Brussels, 18 January 1999.
2. European Chemicals Agency, bd51087b-9917-b018-69ac-d7594315e2a9
3. Chinyere N. Nnaji, Kristina C. Williams, Jonathan M. Bishop, Guido F. Verbeck (2015) Science and Justice 55: 162-167.