Combinational Protein Library Created by Researchers
Researchers from the University of Leeds have created a new protein library which details the ways in which proteins interact with each other.
In a study published in the European Journal of Organic Chemistry, the researchers detail the developement of a combinatorial library which can be used to screen supramolecular ligand structures which could disrupt protein-protein interaction.
This type of combinatorial chemistry is popular within the pharmaceutical industry as it allows chemists to test large amounts of molecules for desirable properties. However, protein surfaces are large three-dimensional structures which contain areas of different acidity, basicity and hydrophobicity, found in different positions on their surface. This means that the screening of inhibitors of protein–protein interactions must involve more complex ligands (ions or molecules which bind to a central metal atom to form a coordination complex) than those needed for active site recognition of desirable properties. Although proteins prefer a neutral pH, reversible hydrazone-forming chemistry usually takes place at an acidic pH so the scientists had to come up with a creative solution; the addition of an aniline-based catalyst. This addition pushed the hydrazone exchange reaction towards thermodynamic equilibrium at close to neutral pH.
With this solution in place the scientisits chose a tetraphenylporphyrin scaffold bearing four hydrazides and substituted benzaldehydes as recognition arms that would reversibly link with the hydrazides to form hydrazones, allowing the researchers to facilitate protein surface recognition.
With the first set up of the model combinatorial protein library the researchers tested two substituted aldehyde ligands, and these formed an equilibrium with the benzaldehyde-derived hydrazone. Mass Spectrometry analysis revealed that the product distribution was at thermodynamic equilibrium, and the ligands exchanged by reversible reaction. This meant that the conditions for a dynamic combinatorial library for protein surface recognition were set.
Mass Spectrometry will be important for the future development of the protein library as this analytical technique ionizes chemical species and sorts ions based on their mass-to-charge ratio, making it possible to check if other ligand combinations exchange by reversible reaction.
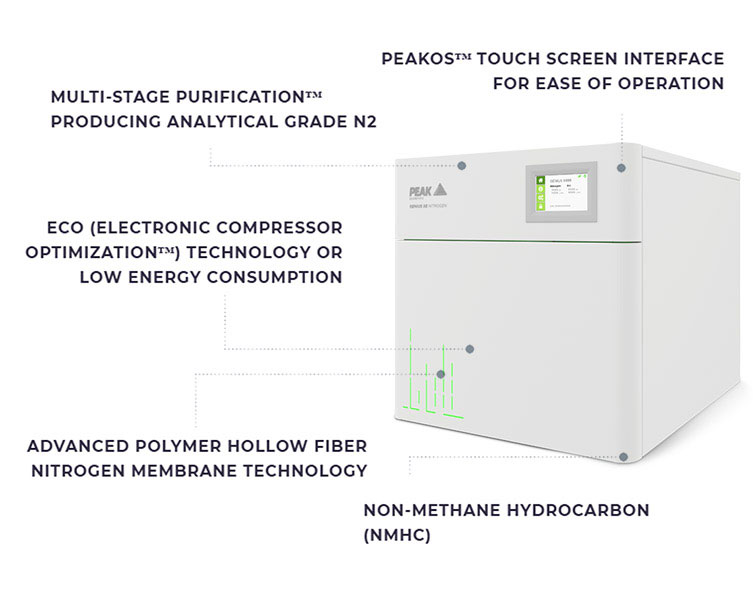
At Peak Scientific we know how important it is to ensure that your gas supply for Mass Spectrometry is reliable, consistent and of a certifiable purity, which is why we have developed a range of nitrogen gas generators specifically suited to this application.
Our latest product innovation, Genius XE Nitrogen, provides a premium standalone nitrogen solution for high performance LC-MS and other mission-critical laboratory applications where performance and reliability are paramount. Genius XE delivers factory certifiable purity up to 99.5% on-demand, 24/7, so you can you can be assured that your instrument is receiving a constant supply of the high purity gas it requires. If you would like further information about Genius XE or any of our hydrogen, nitrogen or zero aire gas genetators please contact us.
Features of Genius XE
Find more information about the combinational protein library.
Found this article interesting? You might also like:
Breakthrough in bacterial antibiotic immunity research
User Story: Maimonides Institute for Biomedical Research of Cordoba
Alternative carrier gas for GC and GC-MS
Source