Detailed hydrocarbon analysis (DHA) is a separation technique used by a variety of laboratories involved in the petrochemical industry for analysis and identification of individual components as well as for bulk hydrocarbon characterisation of a particular sample. Bulk analysis looks at gasoline composition in terms of PONA components (Paraffins, Olefins, Naphthalenes and Aromatics) and other fuels in the C1-C13 range since this gives an indication of overall quality of the sample.
The hydrogen trace generator used in this study was the Peak Precision 500 Hydrogen Trace Generator.
The analysis of gasoline for spark ignition components is essential for quality control. Owing to the complex nature of gasoline samples, good resolution between eluents is required and therefore a long column is used (typically 100m). Several methods are routinely used for DHA which differ in their oven temperature ramp rates or in the length of column used. Each method has its advantages and disadvantages since some improve peak resolution of low boiling compounds whereas others provide better resolution of heavier compounds at the end of the chromatogram. The complex nature of the methodology coupled with the use of such a long column means that run times can easily exceed 120 minutes when using helium carrier gas. However, the use of hydrogen can vastly increase run rates because of its efficiency at higher linear velocities. This is a particularly attractive prospect for oil analysis laboratories since faster throughput of sample means increased profitability. The benefits of using hydrogen in terms of improved chromatography combined with the increasing cost of helium along with supply issues means that laboratories switching from helium to hydrogen can become much more profitable whilst maintaining standards of analysis that conform to industry standards.
This application note demonstrates a comparison of gasoline analysis using helium carrier gas following ASTM method D67291 and the use of unfiltered hydrogen carrier gas produced by a Peak Scientific Precision Trace hydrogen generator in DHA following ASTM method D6729- 1 appendix X2 and demonstrates the improvement in run time whilst maintaining crucial separations between certain components.
Results and discussion
Detailed hydrocarbon analysis of gasoline showed that the elution time of the last compound in the mixture, n-Pentadecane, could be reduced from 125 minutes to less than 74 minutes by switching carrier gas from helium to hydrogen (figure 1). Despite the difference in analysis times, the PONA analysis showed that quantitative differences were not significantly different when using either carrier gas (table 1). Despite the much higher carrier gas flow rates when using hydrogen carrier gas, critical separations were still achieved in most cases and in certain cases were even improved. Separation of 1-methylcyclopentene and benzene, which is highly regulated analysis because of the importance of the benzene fraction, was actually improved when using hydrogen carrier gas despite the quicker elution times of the compounds with hydrogen as a carrier gas (figure 2). Separation of Toluene and 2,3,3-Trimethylpentane was achieved using helium whereas with hydrogen the two compounds co-eluted (figure 3). To separate these two compounds using hydrogen carrier gas some improvements to the method would need to be made. Separation of Tridecane and 1-methylnaphthalene was achieved equally well using both carrier gases (figure 4).
The results of the DHA show that the use of hydrogen as a carrier gas, following ASTM D6729 appendix X2 methodology can vastly reduce analysis times for gasoline analysis whilst providing the necessary resolution required for separations of critical components.
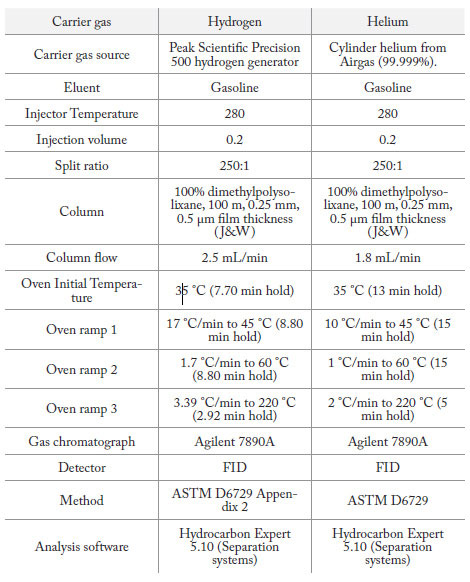
Table 1: Designation D 6729-01 Standard Test Method for Determination of Individual Components in Spark Ignition Engine Fuels by 100 Meter Capillary High Resolution Gas Chromatography. ASTM International 2002.
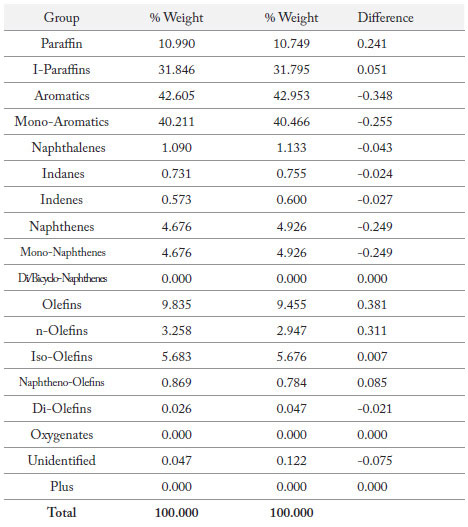
Table 2. Quantitative results of PONA compounds.
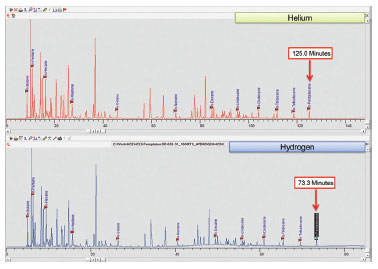
Fig 1. Comparison of DHA of total gasoline sample using hydrogen and helium.
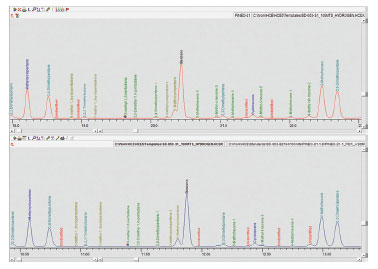
Fig 2. Comparison of separation of 1-methylcyclopentene and benzene when using hydrogen and helium as carrier gas.
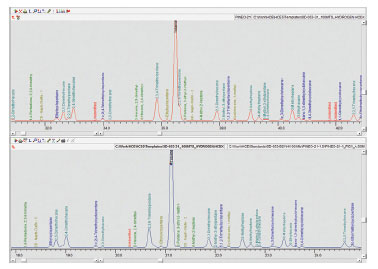
Fig 3. Comparison of separation of Toluene and 2,3,3-Trimethylpentane when using hydrogen and helium as carrier gas.
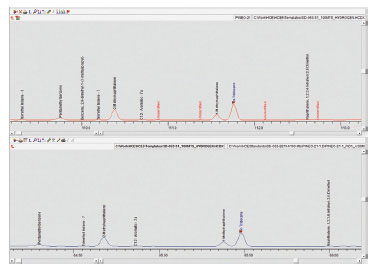
Fig 4. Comparison of separation of Tridecane and 1-methylnaphthalene when using hydrogen and helium as carrier gas.

Ed Connor DR.SC. is GC-MS Application Specialist, Peak Scientific, Inchinnan Business Park, Scotland, UK . Prior to joining Peak in February 2013, Ed completed his Dr.Sc. at ETH Zurich in Switzerland using GC-MS to look at herbivore induced plant volatiles and their interaction with beneficial insects. He then joined the University of Zurich where his work focused primarily on volatile collection methods and analyses using GC-MS and GC-FID . +44 141 812 8100, econnor@peakscientific.com
Download File
References
1. Designation D 6729-01 Standard Test Method for Determination of Individual Components in Spark Ignition Engine Fuels by 100 Meter Capillary High Resolution Gas Chromatography. ASTM International 2002.
2. Designation D6729 – 01 Appendix X2. Hydrocarbon data using hydrogen carrier. ASTM International 2004.
To find out more about Peak Scientific's hydrogen, nitrogen and zero air Precision series generators for GC click here or contact us.